Treating metastatic or advanced prostate cancer
Prostate cancer cells need androgen hormones, such as testosterone, to grow.5 In the early stages of the disease, surgical castration or androgen deprivation therapy (ADT) is utilized to decrease androgen production and inhibit the growth of prostate cancer cells. However, it is important to note that a considerable number of prostate cancer cases eventually advance despite surgical castration or ADT, as the adrenal glands continue to produce a certain amount of testosterone.14,15 Hormone therapies are a part of the standard of care for metastatic prostate cancer early lines and aim to reduce testosterone levels or to directly block it from fueling prostate cancer cell growth.5,6
Sometimes, despite castrate levels of testosterone achieved by surgical castration or medical castration with ADT,16 hormone therapies are no longer effective at reducing or blocking testosterone levels, and patients develop what is referred to as castration-resistant prostate cancer (CRPC), which has increased disease burden and worse survival outcomes.5-7
Castration-resistant prostate cancer (CRPC) develops through three primary mechanisms: AR amplification, AR bypass, and alterations in steroidogenesis-related enzymes.17
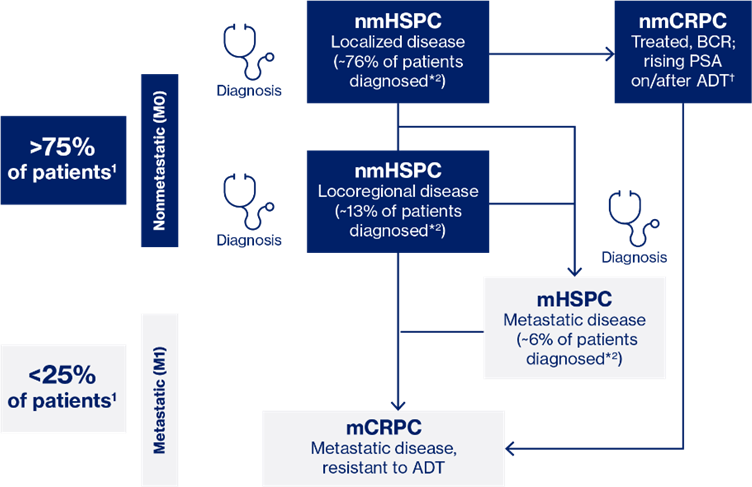
About 10 to 20% of men with prostate cancer develop CRPC within 5 years of diagnosis7 while the majority of men with metastases at diagnostsis progress to mCRPC within a median of~1 year when treated with ADT alone.19-21
It is common for CRPC to exhibit distant metastases, with approximately 84% of men initially diagnosed with metastatic disease. The presence of bone metastases poses a significant challenge, as it often results in substantial morbidity due to skeletal-related events (SREs) and higher mortality rates.7 Additionally, the prognosis for individuals with visceral metastases, particularly in the liver, is notably grim.25,26
Patients with mCRPC experience a steep decline in quality of life
mCRPC (Metastatic Castration-Resistant Prostate Cancer) can have a significant negative impact on the quality of life (HRQoL) of patients. Various sensitive domains are affected, including pain, nausea and vomiting, dyspnea, and appetite loss, which can greatly affect the well-being of individuals with mCRPC.27 Patients with mCRPC also commonly experience intense fatigue and a decline in various areas, particularly physical functioning.28,29 The most significant symptoms for men with mCRPC often involve urinary, hormonal, fatigue, pain/stiffness, and sexual dysfunction.28,30 Additionally, the emotional toll of the disease can be considerable, with common impacts such as depression, frustration, anxiety, and stress.28,30
OVERVIEW OF PC TREATMENT LANDSCAPE
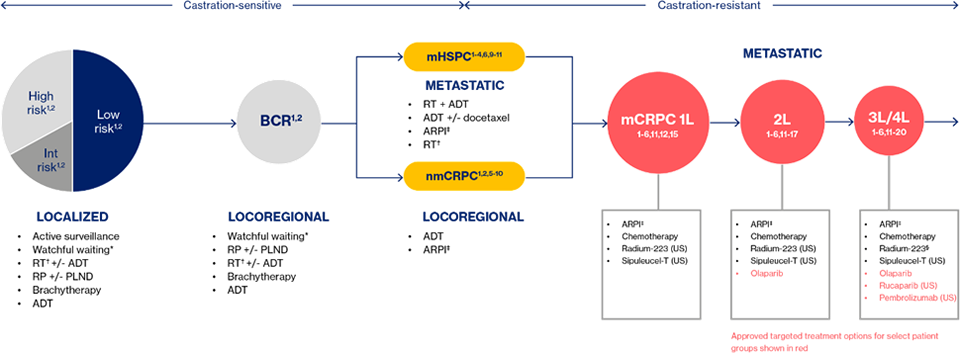
Most treatment options for mCRPC are non-targeted and are associated with modest survival benefits, along with off-target effects and toxicity that can compromise QoL.31–33 These limitations in treatment choices for mCRPC are often a result of their prior use, as well as their utilization in earlier prostate cancer settings and the development of treatment resistance, which further reduces their effectiveness and leaves patients with even fewer options.31,32
Although the treatment landscape is evolving slowly towards precision medicine34,35, non-targeted, systemic treatment still form the current SoC for most men with mCRPC.
The non-targeted, systemic treatment in mCRPC setting are mainly hormonal therapy such as the androgen receptor pathway inhibitors (e.g; Enzalutamide, Abiraterone) and Taxane type chemotherapy (e.g; Docetaxel, Cabacitaxel) from which treatment decisions and sequencing are complicated36, expressing a need for patient profiling for suitable targeted therapy.10,37
A. ADT
Androgen deprivation therapy (ADT) for prostate cancer involves various approaches such as orchiectomy, LHRH agonists, LHRH antagonists, and AR blockers like flutamide.14 However, many cases that initially respond to ADT eventually relapse to castration-resistant prostate cancer (CRPC) due to several factors14:
- Increased production of androgen receptor (AR) molecules within the tumor cells
- Changes in the AR gene leading to the production of a more active protein
- Alterations in the activities of proteins that regulate AR function
- Cancer growth through mechanisms independent of the AR
While two trials have shown marginal survival benefits for patients continuing LHRH analogs during second- and third-line therapies38,39, all subsequent treatments have been studied in trials involving men receiving ongoing androgen suppression.40 Despite the minimal risk of treatment, the modest potential benefits of continuing ADT outweigh this risk.40
B. ARPI
The development of castration-resistant prostate cancer (CRPC) is inevitable once a patient is on Androgen Deprivation Therapy (ADT).40 To counteract ADT resistance, Androgen Receptor Pathway Inhibitors (ARPIs) have been developed.40 These ARPIs extend survival times and improve the quality of life for patients with advanced prostate cancer.41-45
There are four second-generation, AR-targeted therapies that are indicated for the treatment of advanced prostate cancer. The different mechanisms of actions are:
- Inhibitor of androgen biosynthesis via CYP17 enzyme42,43
- AR signaling inhibitor blocks several steps in the AR pathway 44,45
- AR inhibitor binds directly to the ligand-binding domain of AR46,47,48,49
C. Chemotherapy
Two taxane-based antineoplastic chemotherapies are indicated for metastatic prostate cancer.
- You have for first-line chemotherapy: MoA; disrupts the microtubular networks essential for mitotic and interphase cellular functions in cells50,51 mode of delivery IV. 50,51
- For second-line chemotherapy: MoA: disrupts the microtubular networks essential for for mitotic and interphase cellular functions in cells.50,51 Activity in first-line resistant cancers, Mode of delivery slow IV infusion over 60 minutes.50,52
PARP inhibitors & PD-1/PD-L1 inhibitors
Currently approved targeted therapies for men with metastatic castration-resistant prostate cancer (mCRPC) have certain limitations, restricting their applicability to a subset of patients.52-55 Approximately 19% to 23% of men with mCRPC display DNA repair pathway alterations, making them suitable candidates for targeted therapies like PARP inhibitors.56 However, this percentage represents only a fraction of the overall patient population. The use of PD-1/PD-L1 inhibitors in progressive mCRPC has even less coverage.57,58 Additionally, only about 3% to 5% of men with mCRPC have MSI-H/dMMR disease*, a specific type of disease associated with high microsatellite instability and deficient DNA mismatch repair. The implementation of targeted therapies for these patients is further hindered by the requirement for highly accurate testing to identify specific genetic alterations and biomarkers. As a result, the global usage of these targeted therapies is limited.
(*) Based on targeted sequencing analysis of a case series of 1551 tumors from 1346 patients with PC
Approved targeted mCRPC treatments lack universal coverage56,57 |
|
PARP inhibitors52-55 |
PD-1/PD-L1 inhibitors57-59 |
|
Therapeutic target: PARP |
Therapeutic target: PD-1/PD-L1 |
|
Target patient population: |
Target patient population: |
|
~19%–23% of men with mCRPC56 |
~3%–5% of men with mCRPC*57,58 |
PARP Inhibitors
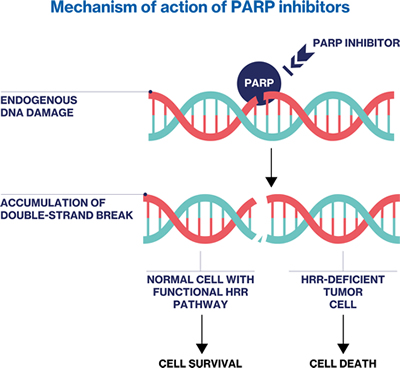
PARP inhibitors are targeted therapies that specifically focus on tumors with DNA damage repair defects. PARPs play a crucial role in the efficient repair of single-strand DNA breaks.52-55 By preventing the dissociation of PARP from DNA, PARP inhibitors block the DNA damage response (DDR) pathway.52-55 During cell replication, the interaction between the replicating fork and the PARP-DNA adduct generates double-strand breaks. 52-55 In normal cells, the homologous recombination repair (HRR) pathway repairs these breaks.52-55 However, in cells with HRR defects, alternative and error-prone pathways are activated, resulting in increased genomic instability and cell death. 52-55 It's important to note that germline mutations in HRR-associated genes are found in only about 10% of men with mCRPC, while another 20% have somatic mutations.56-60
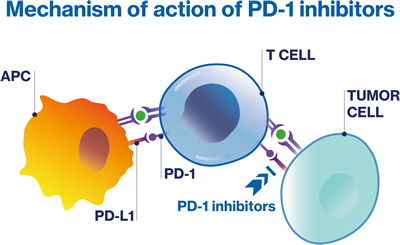
PD-1/PD-L1 inhibitors
PD-1/PD-L1 inhibitors are a form of immunotherapy that work by releasing the PD-1 suppression of the immune response. Immunotherapy, in general, enhances the body's natural defenses to fight against cancer;61 Specifically, pembrolizumab is a PD-1 inhibitor that blocks the interaction between PD-L1 and PD-1, thereby releasing the PD-1 pathway-mediated inhibition of the immune response.59,62 The efficacy of pembrolizumab has been demonstrated in patients with MSI-H or dMMR solid tumors, including prostate cancer.59,62 It is important to note that approximately 3% to 5% of prostate cancer patients have dMMR, which involves mutations in genes such as MSH2, MSH6, PMS2, and MLH1.57,58 These mutations typically lead to hypermutation and microsatellite instability (MSI).
Immunotherapy: Sipuleucel-T
The utility of sipuleucel-T, a therapeutic option for metastatic castration-resistant prostate cancer (mCRPC), has been limited due to various factors. Compared to other available therapies, sipuleucel-T may not be widely used.63 Four main factors have affected the early adoption of sipuleucel-T.63 These include the initial delay in FDA approval, the reimbursement delay after approval, the investment required for practices to offer sipuleucel-T, and the emergence of other non-chemotherapy treatments. Although studies of sipuleucel-T did not involve traditional endpoints like PSA levels,63 it was found that the time to first opiate use was significantly improved in treated patients compared to the control group.64 This is significant because pain can have a substantial impact on survival time.65,66 Despite these potential benefits, a database analysis revealed that only 10% of patients who received treatment for mCRPC between 2010 and 2016 received sipuleucel-T.63 However, it is possible that the delayed treatment response of sipuleucel-T is now more accepted, particularly in the "era of immunotherapy".64
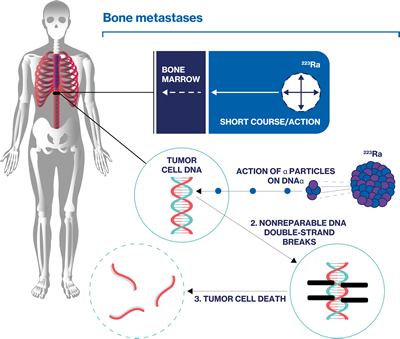
Bone-directed treatment: 223Ra dichloride
223Ra dichloride |
|
|
Mechanism of action |
|
|
Mode of delivery |
iv69,70 |
Radionuclide therapy such as 223Ra dichloride is indicated for both palliation and extension of survival in mCRPC with bone metastases.67,68
Radioligand therapy (RLT)
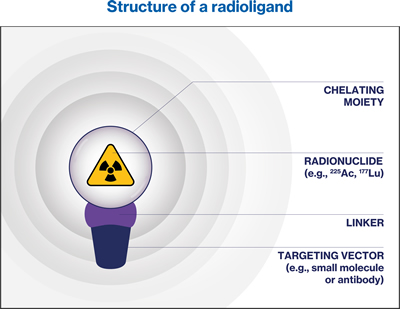
RadioLigand Therapy (RLT) is an innovative approach for cancer treatment that utilizes a targeted radioactive drug, known as a radioligand.72 A radioligand consists of a radioactive atom, called a radionuclide, which is bound to either a cell-targeting antibody or a small molecule known as a ligand. This ligand specifically binds to certain cell-surface molecules found on tumor cells.72 RLT involves the systemic administration of a specific radioligand via injection, allowing for the delivery of cytotoxic radiation directly to the targeted tumor cells.72 By selectively targeting radionuclides to cancer cells, RLT aims to maximize the therapeutic effects on the tumor while minimizing any potential off-target effects.72
A ”see it, treat it” approach
Radioligands can be used for diagnostic imaging and therapeutic applications (together known as theranostic), both of which have the same precision-based approach. This allows healthcare professionals to use radioligand imaging to visualize the target cancer cells and select specific patients eligible for radioligand therapy.75,76
In the context of mCRPC, acquired resistance and side effects of current treatments pose as significant barriers, leading to the development of biomarker-based approaches such as targeting PSMA receptor.6
PSMA as a biomarker
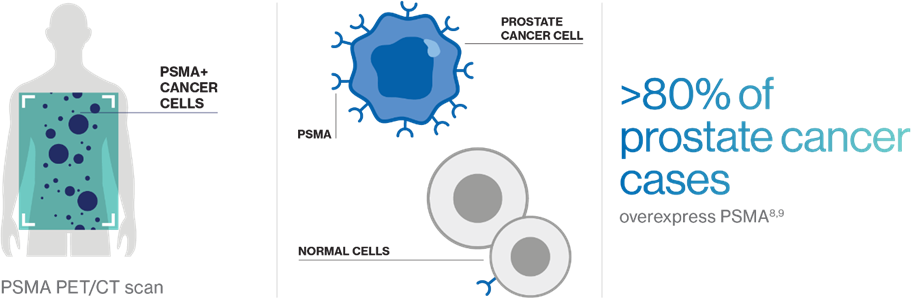
Prostate-specific membrane antigen (PSMA) is a cell-surface protein that is often highly expressed in metastatic prostate cancer relative to normal tissue (e.g., kidneys, salivary glands).8,9
PSMA PET/CT scans can determine PSMA status and visualize where PSMA-positive (PSMA+) cancer cells have spread throughout the body.4,8
More than 80% of prostate cancers are PSMA+, making it an ideal biomarker for physicians to see how the cancer is progressing and determine the course of treatment, including the potential use of PSMA-targeted therapies.4,8
- Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet. 2021;398(10305):1075-1090. doi:10.1016/S0140-6736(21)00950-8
- Desai K, McManus JM, Sharifi N. Hormonal Therapy for Prostate Cancer. Endocr Rev. 2021;42(3):354-373. doi: 10.1210/endrev/bnab002
- Yamada Y, Beltran H. The treatment landscape of metastatic prostate cancer. Cancer Lett. 2021;519:20-29. doi: 10.1016/j.canlet.2021.06.010
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x
- Giraudet AL, Kryza D, Hofman M, et al. PSMA targeting in metastatic castration-resistant prostate cancer: where are we and where are we going? Ther Adv Med Oncol. 2021;13:17588359211053898. doi:10.1177/17588359211053898
- Hupe MC, Philippi C, Roth D, et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front Oncol. 2018;8:623. doi:10.3389/fonc.2018.00623
- Nuhn P, De Bono JS, Fizazi K, et al. Update on Systemic Prostate Cancer Therapies: Management of Metastatic Castration-resistant Prostate Cancer in the Era of Precision Oncology. Eur Urol. 2019;75(1):88-99. doi:10.1016/j.eururo.2018.03.028
- National Cancer Institute. Hormone Therapy for Prostate Cancer Fact Sheet. https://www.cancer.gov/types/prostate/prostate-hormone-therapy-fact-sheet (accessed December 2023)
- Harris P, et al; Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion;. Nat Clin Pract Urol. 2009; 6(2):76–85. Doi: 10.1038/ncpuro1296
- Saad F, et al; 2021 Canadian Urological Association (CUA)-Canadian Uro Oncology Group (CUOG) guideline: Management of castration-resistant prostate cancer (CRPC); Can Urol Assoc J. 2021;15(2):E81–E90. doi: 10.5489/cuaj.7074
- Huang Y, et al; Molecular and cellular mechanisms of castration resistant prostate cancer; Oncol Lett. 2018 May; 15(5): 6063–6076. doi: 10.3892/ol.2018.8123
- Gravis G, et al. Lancet Oncol. 2013;14(2):149–158;
- James ND, et al. Eur Urol. 2015;67(6):1028–1038;
- Sweeney CJ, et al. N Engl J Med. 2015;373:737–746;
- Iwamoto H, et al. J Clin Oncol. 2018;36:6(suppl 291);
- Bubendorf L, et al. Hum Pathol. 2000;31(5):578–583
- Summers N, et al. Curr Med Res Opin. 2017;33:1995–2008;
- Holmstrom S, et al. Patient. 2019;12:57–67;
- Kuppen MCP, et al. Clin Genitourin Cancer 2020;18(3):e233–e253;
- Sullivan PW, et al. Qual Life Res. 2007;16:571–575.
- Shore N, et al. Urology. 2017;109:6–18;
- He L, et al. Medicine (Baltimore). 2020;99(15):e19760;
- Zheng H, et al. Biomed Res Int. 2017;2017:3941217.
- de Bono J, et al. N Engl J Med. 2020;382(22):2091–2102;
- Hofman MS, et al. Lancet Oncol. 2018;19(6):825–833;
- Shore ND. Urology. 2014;83:00
- Hahn AW, et al. Cancer Treat Res Commun. 2019;19:100120;
- Taylor CD, et al. J Clin Oncol. 1993;11:2167–72;
- Hussain M, et al. J Clin Oncol. 1994;12:1868–1875;
- Mottet N, et al. EAU Guidelines: Prostate Cancer. https://uroweb.org/guideline/prostate-cancer/ (accessed March 2024).
- Zhao J, et al. Molec Can Ther. 2020;19(8):1708-1718;
- Zytiga (abiraterone) tablets. SmPC. Janssen-Cilag International NV; May 2016;
- Zytiga (abiraterone) tablets. Prescribing information. Janssen Biotech, Inc; October 2020;
- Xtandi (enzalutamide) capsules. SmPC. Astellas Pharma Europe BV; February 2018;
- Xtandi (enzalutamide) capsules/tablets. Prescribing information. Astellas Pharma US, Inc; August 2020;
- Nubeqa (darolutamide) tablets. SmPC. Bayer AG; March 2020;
- Nubeqa (darolutamide) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals Inc; July 2019;
- Erleada tablets (apalutamide). SmPC. Janssen-Cilag International NV; January 2019;
- Erleada (apalutamide) tablets. Prescribing information. Janssen Pharmaceutical Companies; July 2020.
- Jevtana solution for infusion (cabazitaxel). SmPC. Sanofi-Aventis Deutschland GmbH; November 2015;
- Jevtana (cabazitaxel) injection. Prescribing information. Sanofi-Aventis US LLC; March 2020.
- Lynparza (olaparib) tablets. Prescribing information. AstraZeneca Pharmaceuticals LP; May 2020;
- Lynparza capsules (olaparib). SmPC. AstraZeneca AB; November 2020;
- Rubraca (rucaparib) tablets. Prescribing information. Clovis Oncology Inc; May 2020;
- Rubraca tablets (rucaparib). SmPC. Clovis Oncology Ireland Ltd; March 2019;
- Robinson D, et al. Cell. 2015;161(5):1215–1228;
- Ku S, et al. Nat Rev Urol. 2019;16(11):645–654;
- Abida W, et al. JAMA Oncol. 2019;5(4):471–478;
- Keytruda (pembrolizumab) injection. Prescribing information. Merck Sharp & Dohme Corp; June 2020.
- Pritchard CC, et al. N Engl J Med. 2016;375:443–453.
- American Society of Clinical Oncology. Prostate cancer: types of treatment. https://www.cancer.net/cancer-types/prostate-cancer/types-treatment (accessed January 2021);
- Keytruda powder for infusion (pembrolizumab). SmPC. Merck Sharp & Dohme BV; March 2020;
- Caram ME, et al. JAMA Network Open. 2019;2(4):e192589;
- Small EJ, et al. Prostate Cancer Prostatic Dis. 2014;17:259–264;
- Halabi S, et al. J Clin Oncol. 2008;26:2544–2549;
- Armstrong AJ, et al. Clin Cancer Res. 2007;13:6396–6403.
- Parker C, et al. N Engl J Med. 2013;369(3):213–223;
- Hoskin P, et al. Lancet Oncol. 2014;15(12):1397–1406;
- Xofigo (radium 223 dichloride). Prescribing information. Bayer Healthcare Pharmaceuticals, Inc; December 2019;
- Xofigo (radium 223 dichloride). SmPC. Bayer AG; June 2018;
- Lassmann M, et al. Eur J Nucl Med Mol Imaging. 2013;40(2):207–212.
- Society of Nuclear Medicine and Molecular Imaging. Fact sheet: Targeted radionuclide therapy and prostate cancer. http://www.snmmi.org/AboutSNMMI/Content.aspx?ItemNumber=12772 (accessed November 2020);
- Benešová M, et al. J Nucl Med. 2015;56(6):914–924;
- Jalilian A. Iran J Nucl Med. 2017;25(1):1–10.
- Aboagye EO, Barwick TD, Haberkorn U. Radiotheranostics in oncology: Making precision medicine possible. CA Cancer J Clin. 2023;73(3):255-274. doi:10.3322/caac.21768
- Duan H, Iagaru A, Aparici CM. Radiotheranostics - Precision Medicine in Nuclear Medicine and Molecular Imaging. Nanotheranostics. 2022;6(1):103-117. doi:10.7150/ntno.64141



